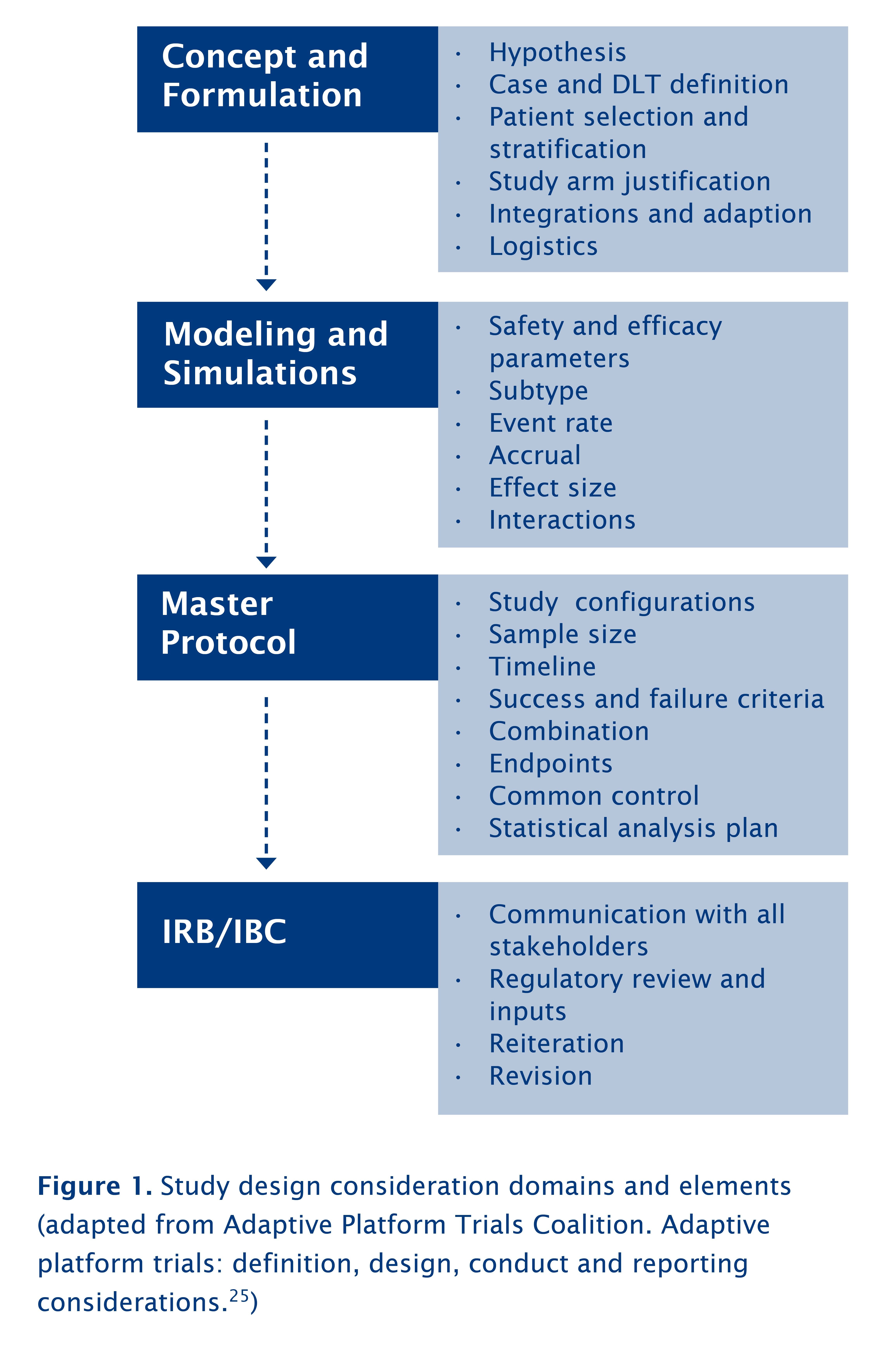
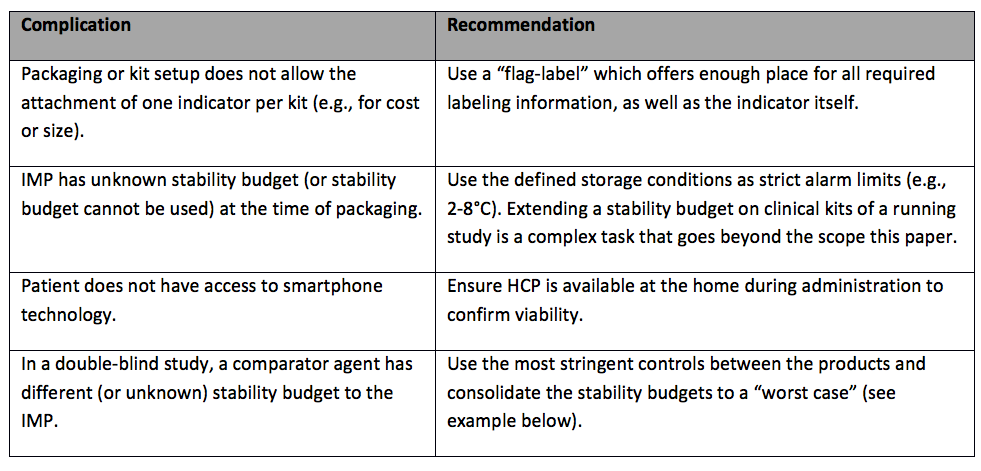
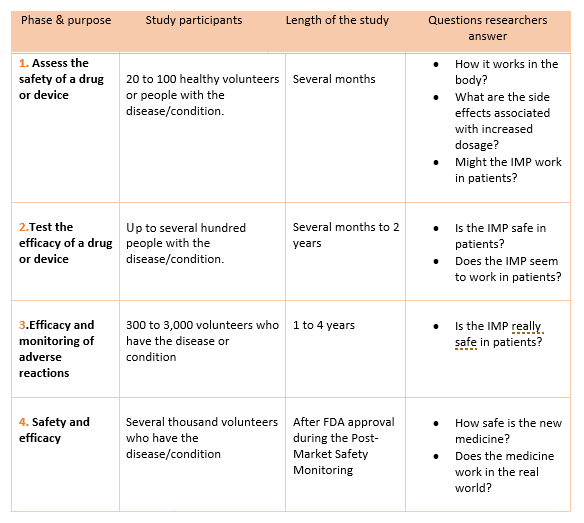
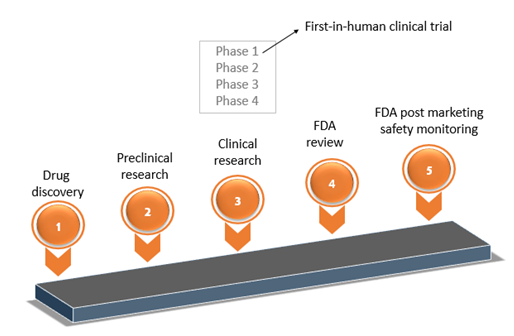
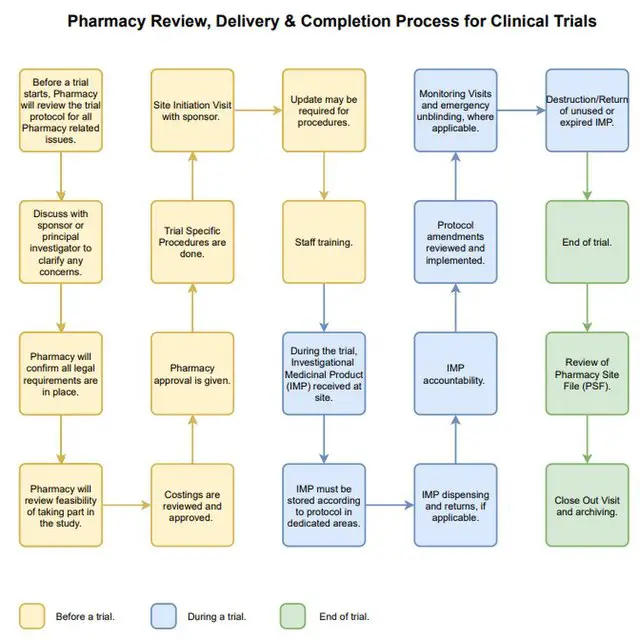
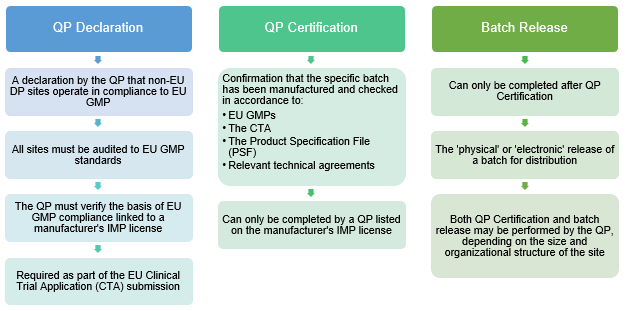
Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs) - PDF Free Download

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download
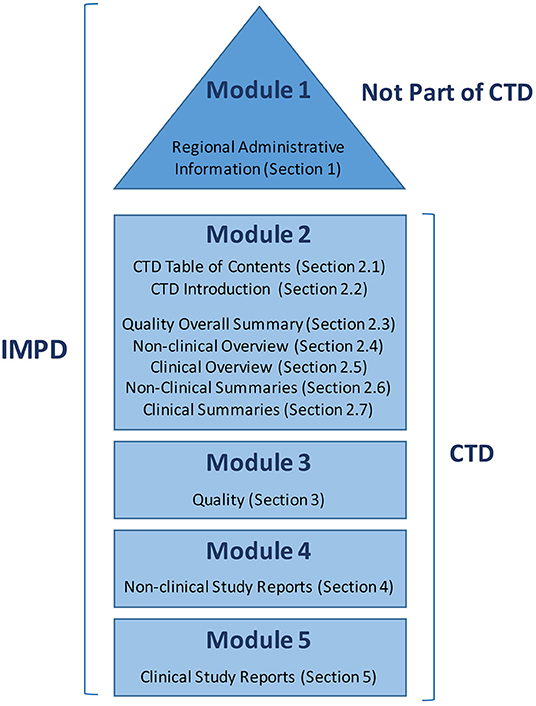
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience | Cardiovascular Medicine