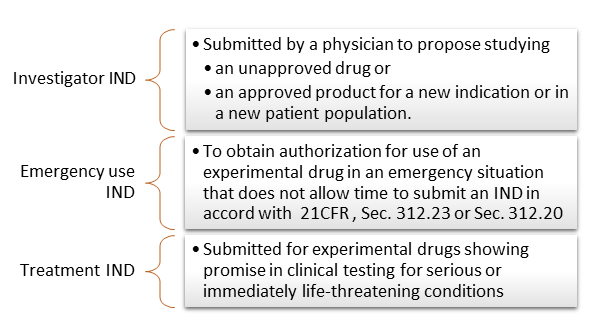
PDF) Consistency of Financial Interest Disclosures in the Biomedical Literature: The Case of Coronary Stents

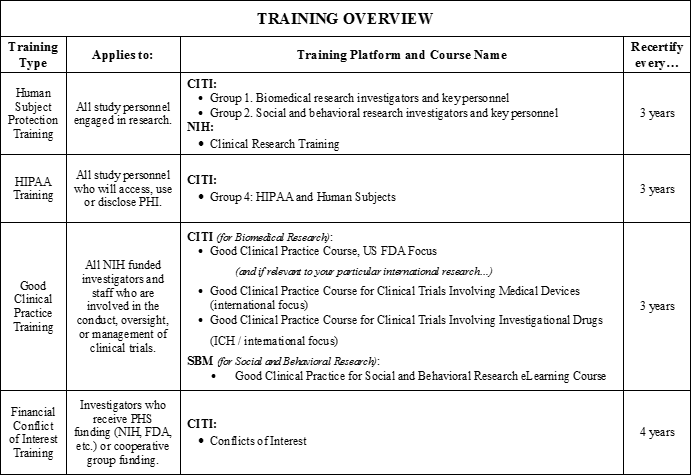
OCR Industry Sponsored Study Guide | Office of Clinical Research | Perelman School of Medicine at the University of Pennsylvania

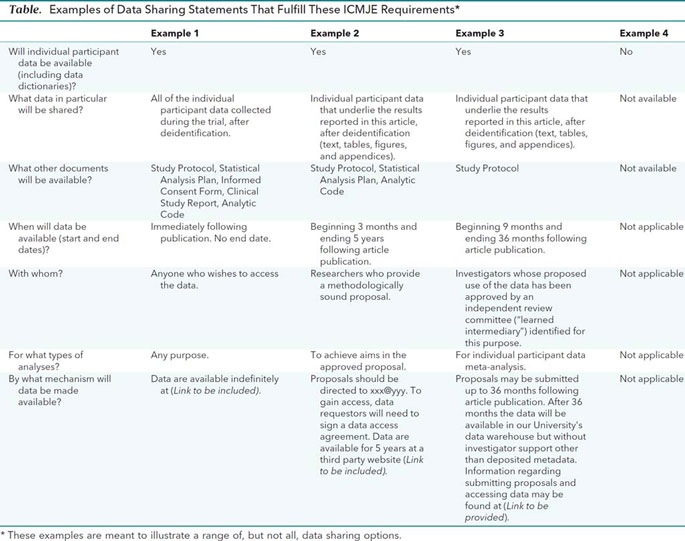
Financial ties of principal investigators and randomized controlled trial outcomes: cross sectional study | The BMJ
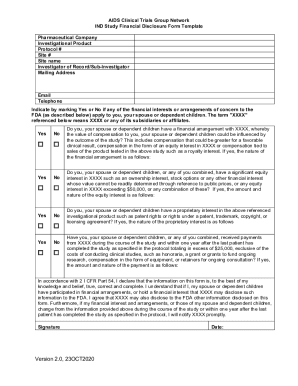
Fillable Online CLINICAL INVESTIGATOR FINANCIAL CERTIFICATION. AIDS Clinical Trials Group Network IND Study Financial Disclosure Form Template Fax Email Print - pdfFiller

Financial disclosure lacking in publication of clinical trials, new research shows | Healthcare Finance News