Book 6: 2021 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC

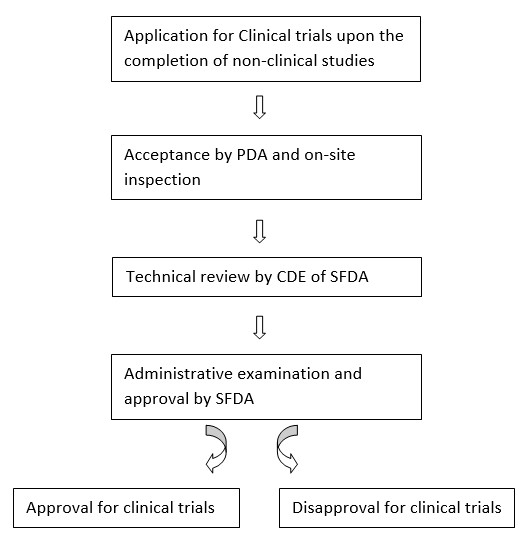
Pharmacovigilance for clinical trials in India: Current practice and areas for reform Brahmachari B, Fernandes M, Bhatt A - Perspect Clin Res

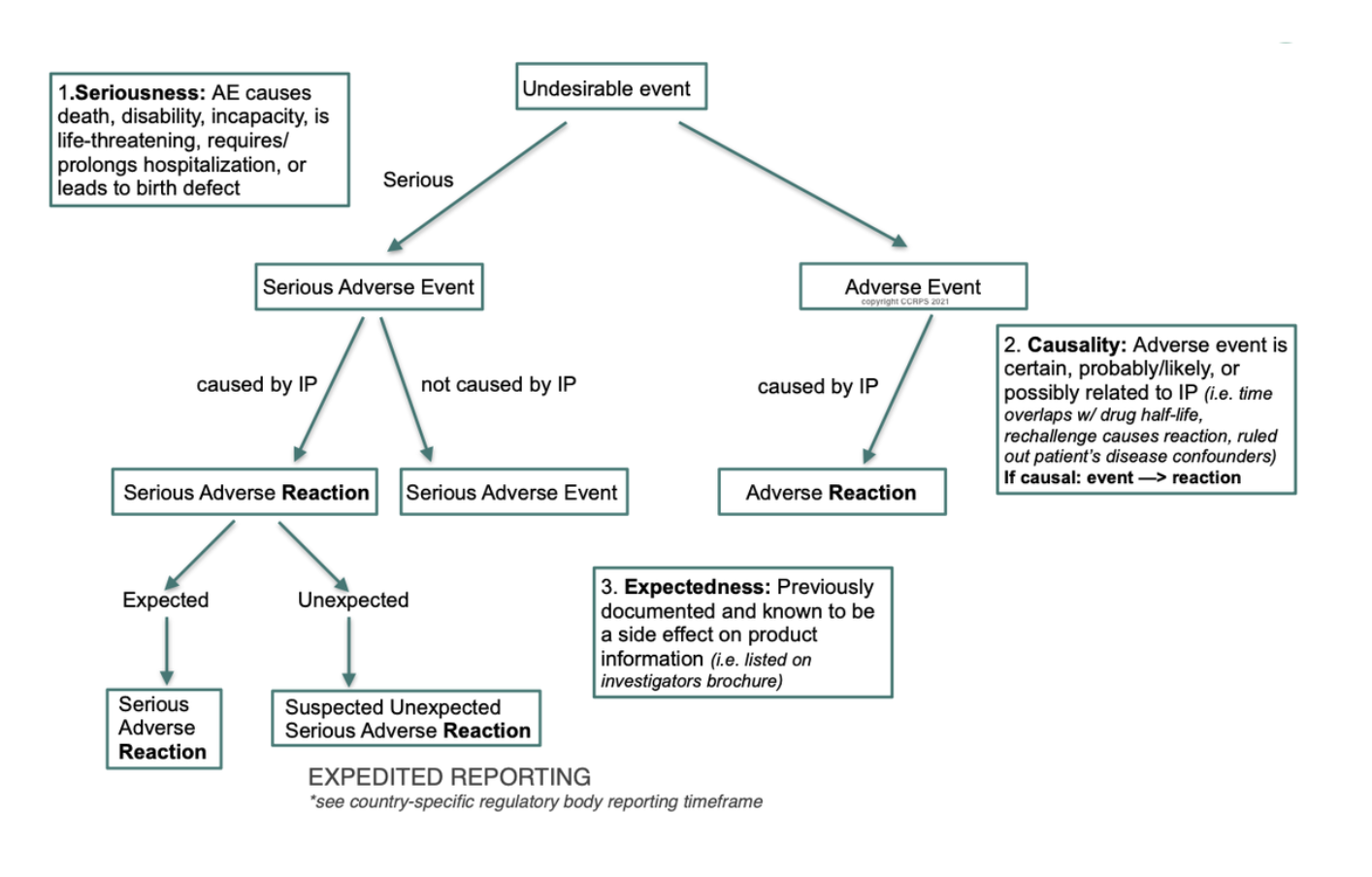
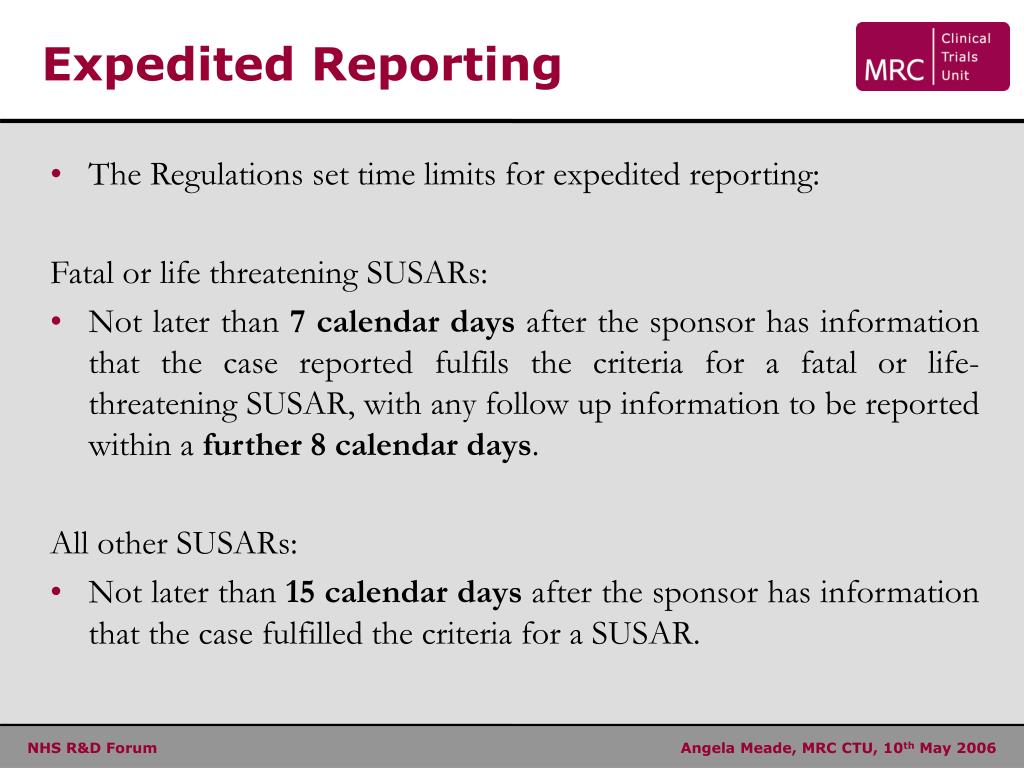
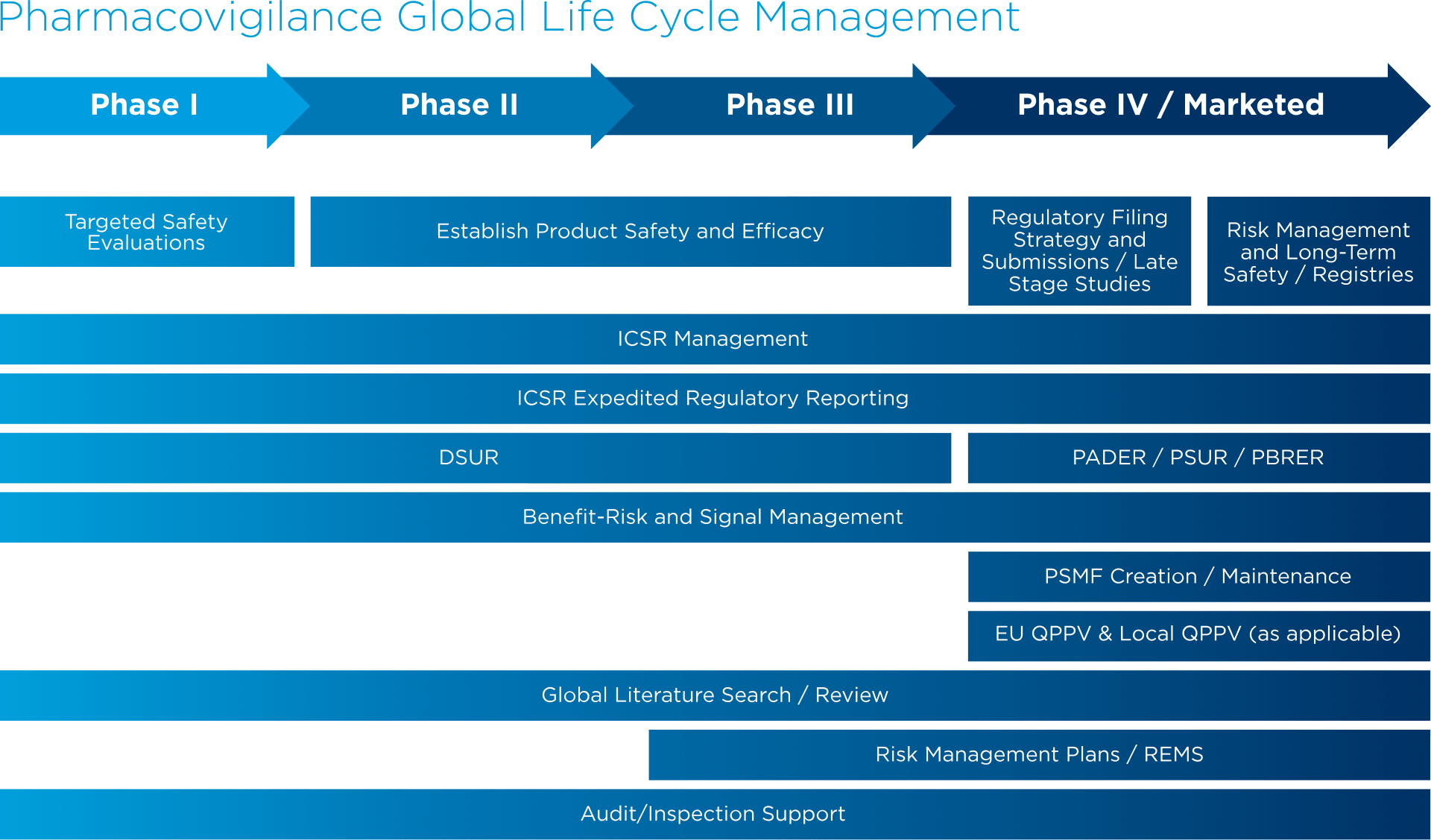
Adverse event (AE) reporting algorithm. Timeframe for adverse event... | Download Scientific Diagram
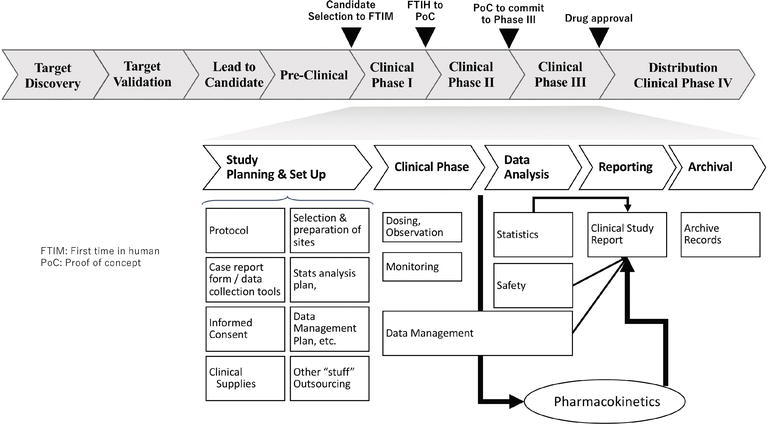
Frameworks for Evaluating Qualitative and Quantitative Information on Adverse Drug Events throughout Development through to Marketing | IntechOpen